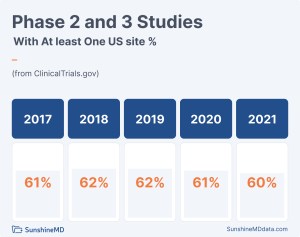
According to the data from ClinicalTrials.gov, the US remains an integral part of all industry sponsored Phase 2 and Phase 3 studies done anywhere under FDA auspices. Despite the rise in clinical trials being performed across the globe, the US remains the largest country for clinical trial sites, often because of the US commercial market’s attractiveness, and almost always because of US medical practices.
Open Payments data is the only federally mandated, validated source of information about US investigators and their enrollment performance on these and other Phase 1, 2 and 3 studies conducted in the US. While it is a great source of information, however, it can be time-consuming and tedious to wade through all the data you need to find proven clinical investigators.
Open Payments requires that all pharmaceutical and medical device companies report all payments to physicians and select other medical professionals by the amount, date, purpose, and company making that payment. The data must be returned to the relevant medical professional for validation. These data include clinical trial research payments to clinical investigators, by name, unique ID number and address. SunshineMD provides this data in a form that allows users to find the most appropriate US investigators for their Phase 1-4 studies.
US Industry Site Activity By Year