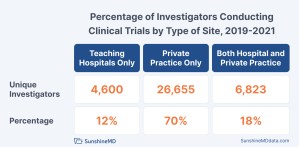
Unlike in many countries outside the United States, clinical trial activity within the US is concentrated in private practices. Almost 70% of clinical investigators conduct all of their clinical trial activity in private practice. Twelve percent (12%) of US clinical investigators conduct all their clinical trial work in affiliated teaching hospitals, of which there are slightly more than 1,000 in the US. The remaining investigators conduct clinical research in both locations.
If you’re looking for experienced US clinical investigators with proven enrollment performance, you’ve found the right blog. Open Payments data is the only federally mandated, validated source of this information.
The Open Payments law requires that all pharmaceutical and medical device companies, with at least one marketed drug, report all payments to physicians and selected other medical professionals by the amount, date, purpose, and company making that payment. The data must be returned to the relevant medical professional for validation. These data include clinical trial research payments to clinical investigators, by name, unique ID number and address.
By using information from both Open Payments and ClinicalTrials.gov, SunshineMD has created a database that can help you find the right US clinical investigators for your clinical trials.
SunshineMD knows study experience and enrollment performance for every one of these investigators at each of these various US sites.