Why SunshineMD?
Our data give a competitive edge by providing information on each active US investigator since 2013. The data include the industry studies in which they participated and enrollment success compared to other investigators.
- There's currently no single source of information that provides US investigator clinical trial experience and enrollment performance.
- SunshineMD is that singular source of information based upon a near census of US clinical investigators.
- Our algorithms and proprietary scoring system make it easy to identify effective sites quickly and easily.
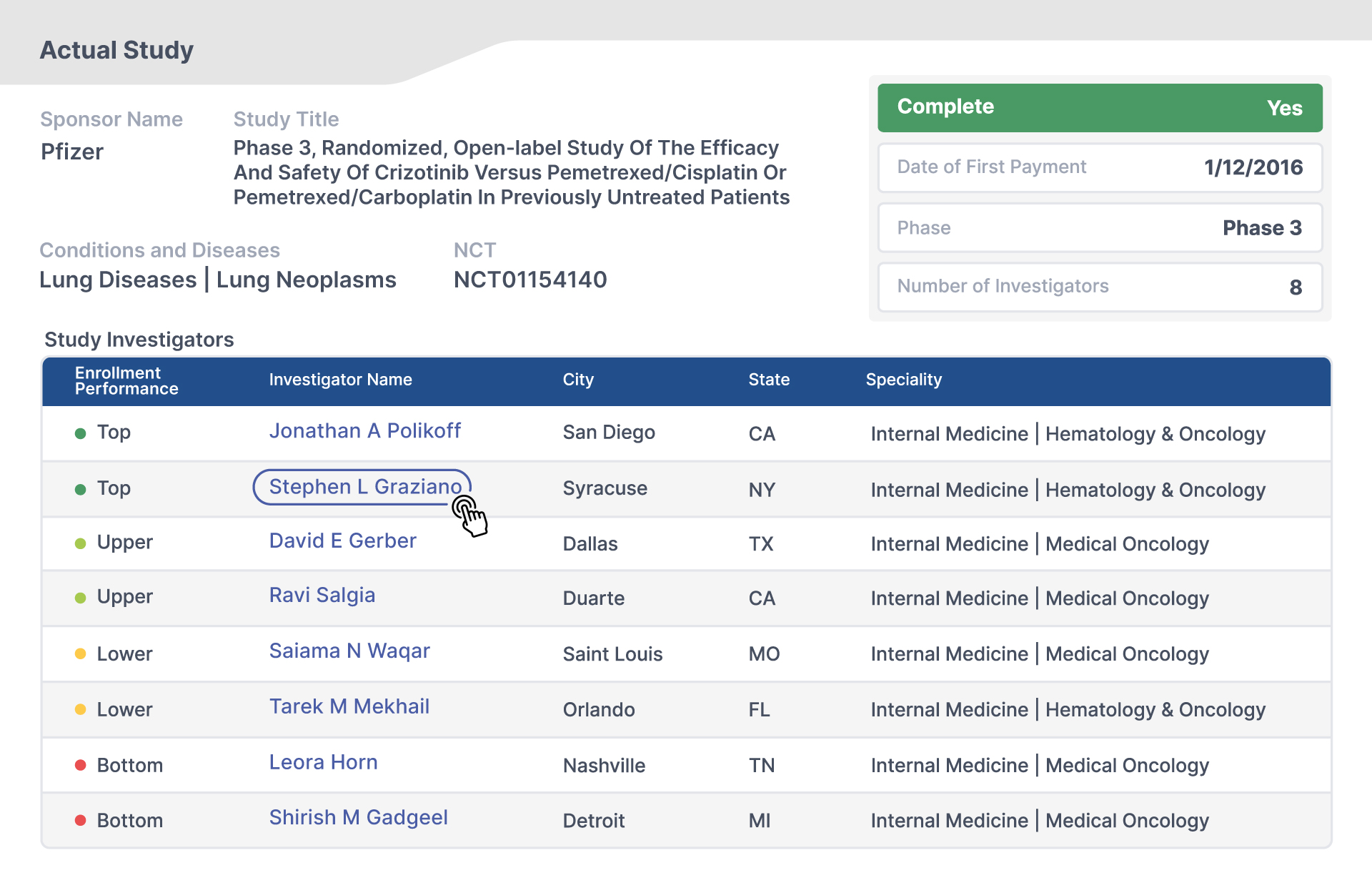
What is the SunshineMD Database?
Documented Data Sources
Sources from Open Payments (CMS) and ClinicalTrials.gov.
Majority of US studies
A vast majority of US clinical trials that occurred between 2013 and 2024.
US investigators
A near census of all investigators, their experience and their comparative enrollment performance.
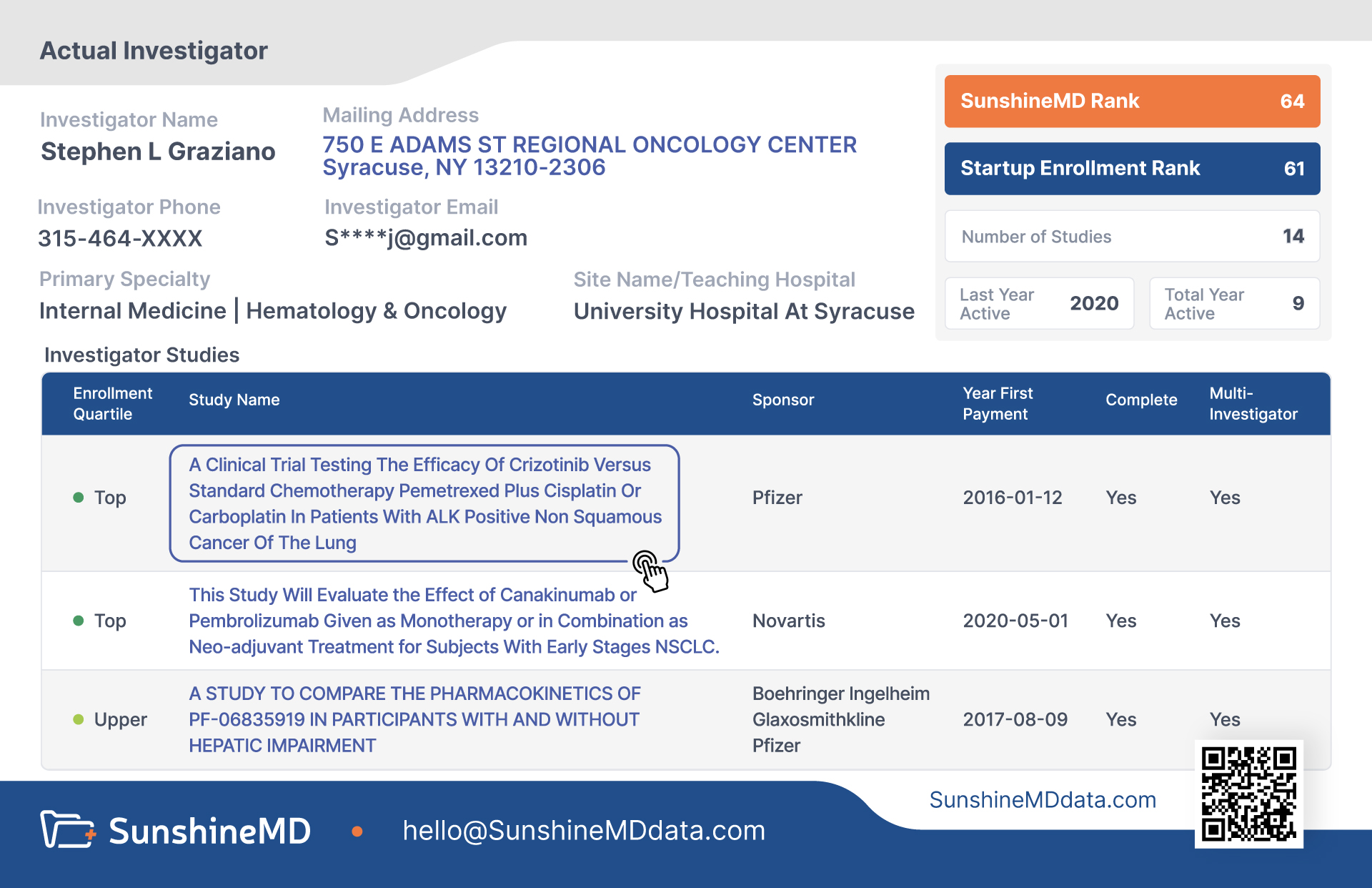
Performance Metrics

- SunshineMD Score
- A summary enrollment measure of an investigator’s overall performance across all the studies on which that investigator has participated in.
- Study Quartile
- An investigator’s enrollment performance in relationship to all the US investigators in any given clinical trial.
- Startup Enrollment Score (for Investigators)
- A summary measure of how quickly an investigator begins enrolling patients into a specific trial.
SunshineMD Results
We confirmed the accuracy of our investigator experience and enrollment performance data by testing our information against actual internal performance data from multiple sponsor companies and CROs.
- Sponsor companies and CROs provided us with studies identified by them. No other detail was provided. We returned the names of all the investigators on those studies, as well as the respective investigators’ comparative enrollment performance.
- The correlation between our data and the actual clinical trial results was consistently .9 and above.
Perspectives
Do Bigger Companies Attract Better Investigators?
A close look at Investigator performance in the Open Payments database shoes that pharmaceutical companies of all sizes are about equally likely to attract top investigators.
Where Are US Clinical Trial Sites Located?
Unlike in many countries outside the United States, clinical trial activity within the US is concentrated in private practices. Almost 70% of clinical investigators conduct all of their clinical trial activity in private practice.
How Many Active Clinical Trial Investigators Exist in the United States?
Do you know how many unique clinical investigators were active on industry sponsored studies over the last three years for which data are available (2018-2020)?
More Clinical Experience Means Better Enrollment Performance
Reviewing the Open Payments database reveals that the investigators with the highest Sunshine Scores are the ones with the most clinical experience.
The US remains the
center of global clinical
trial activity.
According to the data from ClinicalTrials.gov, the US remains an integral part of all industry sponsored Phase 2 and Phase 3 studies done anywhere under FDA auspices.
How Concentrated are US Clinical Trials?
Pharma giants run the most trials and spend the most on research each year. In 2020, the industry's largest companies (in terns of annual spending volume) accounted for more than 74% of total clinical trial spending. Companies throughout the rest of the top 100 barely register in comparison.
About SunshineMD
SunshineMD was born out of a desire to organize a system based in chaos. Founded in 2021 by Dr. Harold E. Glass, Andy Guy, Matthew Morano and Helene Seydoux, SunshineMD is the solution for finding proven US investigators with ease.
In 2021, Dr. Glass teamed up with Mr. Morano, Mr. Guy, and Ms. Seydoux to bring an idea to fruition: using data mandated in the Physician Payments Sunshine Act for a variety of applications. The partners have been able to produce empirically-based performance and experience measures for a near-census of US investigators, allowing those who utilize it to gain market intelligence and maintain a competitive advantage.
Dr. Glass is a retired professor who taught at the University of the Sciences. He has spoken and published widely, particularly in the area of new drug development. He also founded and sold two companies, DataEdge and TTC, llc, serving as a pioneer in the area of clinical trial benchmarking. Many of these data applications continue to be widely used throughout the drug development industry. Some of these applications include GrantPlan and PICAS.
Mr. Morano, Mr. Guy, and Ms. Seydoux bring years of experience in software and marketing technology to the equation. Together, they co-founded Torchlight Technology Group LLC, a digital marketing technology solutions provider for Insurtech companies, which was later sold to Independence Holding Company (IHC).
Can We Help?
If you have any specific questions please do not hesitate to contact us by completing this form.